Author: Karen Seiter, MD, Professor, Department of Internal Medicine, Division of Oncology/Hematology, New York Medical College
Overview:
Introduction
Background
Acute lymphoblastic leukemia (ALL) is a malignant (clonal) disease of the bone marrow in which early lymphoid precursors proliferate and replace the normal hematopoietic cells of the marrow. Acute lymphoblastic leukemia (ALL) may be distinguished from other malignant lymphoid disorders by the immunophenotype of the cells, which is similar to B- or T-precursor cells. Immunochemistry, cytochemistry, and cytogenetic markers may also aid in categorizing the malignant lymphoid clone.
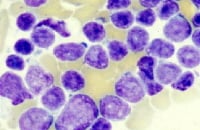
The image below shows pre – B-cell ALL.
The image below shows pre – B-cell ALL.
Diagnostic workup of a patient with pre–B-cell acute lymphoblastic leukemia. Bone marrow aspiration revealed French-American-British L2 morphology.
For excellent patient education resources, visit eMedicine's Blood and Lymphatic System Center and Cancer and Tumors Center. Also, see eMedicine's patient education article Leukemia.
Pathophysiology
The malignant cells of acute lymphoblastic leukemia (ALL) are lymphoid precursor cells (ie, lymphoblasts) that are arrested in an early stage of development. This arrest is caused by an abnormal expression of genes, often as a result of chromosomal translocations. The lymphoblasts replace the normal marrow elements, resulting in a marked decrease in the production of normal blood cells. Consequently, anemia, thrombocytopenia, andneutropenia occur to varying degrees. The lymphoblasts also proliferate in organs other than the marrow, particularly the liver, spleen, and lymph nodes.
Frequency
United States
Acute lymphoblastic leukemia (ALL) is the most common type of leukemia in children. In adults, it is less common than acute myelogenous leukemia (AML). In the United States, approximately 1000 new cases of acute lymphoblastic leukemia (ALL) occur in adults each year.
International
The highest incidence of acute lymphoblastic leukemia (ALL) occurs in Italy, the United States, Switzerland, and Costa Rica.
Mortality/Morbidity
Only 20-40% of adults with acute lymphoblastic leukemia (ALL) are cured with current treatment regimens.
Sex
Acute lymphoblastic leukemia (ALL) is slightly more common in men than in women.
Age
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. However, due to the fact that there are more adults than children, the number of cases seen in adults is comparable to that seen in children.
Clinical
History
- Patients with acute lymphoblastic leukemia (ALL) present with either (1) symptoms relating to direct infiltration of the marrow or other organs by leukemic cells or (2) symptoms relating to the decreased production of normal marrow elements.
- Fever is one of the most common symptoms of acute lymphoblastic leukemia (ALL).
- Patients with acute lymphoblastic leukemia (ALL) often have decreased neutrophil counts, regardless of whether their total white blood cell (WBC) count is low, normal, or elevated. As a result, they are at increased risk of infection. The prevalence and severity of infections are inversely correlated with the absolute neutrophil count (ANC), which is defined as the number of mature neutrophils plus bands per unit of volume. Infections are common when the absolute neutrophil count is less than 500/µL, and they are especially severe when it is less than 100/µL.
- Patients with acute lymphoblastic leukemia (ALL) often have fever without any other evidence of infection. However, in these patients, one must assume that all fevers are from infections until proven otherwise, because a failure to treat infections promptly and aggressively can be fatal. Infections are still the most common cause of death in patients undergoing treatment for ALL.
- Symptoms of anemia are common and include fatigue, dizziness, palpitations, and dyspnea upon even mild exertion.
- Other patients present with signs of bleeding. Bleeding can be the result of thrombocytopenia due to marrow replacement. Additionally, approximately 10% of patients with acute lymphoblastic leukemia (ALL) have disseminated intravascular coagulation (DIC) at the time of diagnosis. These patients may present with hemorrhagic or thrombotic complications.
- Some patients present with palpable lymphadenopathy. Others, particularly those with T-cell ALL, present with symptoms related to a large mediastinal mass, such as shortness of breath.
- Infiltration of the marrow by massive numbers of leukemic cells frequently manifests as bone pain. This pain can be severe and is often atypical in distribution.
- Uncommonly (10-20%), patients may present with left upper quadrant fullness and early satiety due tosplenomegaly.
- Although patients may present with symptoms of leukostasis (eg, respiratory distress, altered mental status) because of the presence of large numbers of lymphoblasts in the peripheral circulation, leukostasis is much less common in people with ALL than those with AML, and it occurs only in patients with the highest WBC counts (ie, several hundred thousand per μL).
- Patients with a high tumor burden, particularly those with severe hyperuricemia, can present in renal failure.
Physical
- Patients with acute lymphoblastic leukemia (ALL) commonly have physical signs of anemia, including pallor and a cardiac flow murmur.
- Fever and other signs of infection, including lung findings of pneumonia, can occur. Fever should be interpreted as evidence of infection, even in the absence of other signs.
- Patients with thrombocytopenia usually demonstrate petechiae, particularly on the lower extremities. A large number of ecchymoses is usually an indicator of a coexistent coagulation disorder such as DIC.
- Signs relating to organ infiltration with leukemic cells and, to a lesser degree, lymphadenopathy may be present.
- Occasionally, patients have rashes that result from infiltration of the skin with leukemic cells.
Causes
- Less is known about the etiology of acute lymphoblastic leukemia (ALL) in adults compared with AML. Most adults with ALL have no identifiable risk factors.
- An increased prevalence of acute lymphoblastic leukemia (ALL) was noted in survivors of the Hiroshima atomic bomb but not in those who survived the Nagasaki atomic bomb. Most leukemias occurring after exposure to radiation are AML rather than ALL.
- Rare patients have an antecedent hematologic disorder (AHD) such as myelodysplastic syndrome (MDS)that evolves to acute lymphoblastic leukemia (ALL). However, most patients with MDS that evolves to acute leukemia develop AML rather than ALL.
- Increasingly, cases of acute lymphoblastic leukemia (ALL) with abnormalities of chromosome band 11q23 following treatment with topoisomerase II inhibitors for another malignancy have been described. However, most patients who develop secondary acute leukemia after chemotherapy for another cancer develop AML rather than ALL.
Differential Diagnosis and Workup:
Differential Diagnosis
Acute Myelogenous Leukemia
Lymphoma, B-Cell
Lymphoma, High-Grade Malignant Immunoblastic
Lymphoma, Mantle Cell
Lymphoma, Non-Hodgkin
Lymphoma, B-Cell
Lymphoma, High-Grade Malignant Immunoblastic
Lymphoma, Mantle Cell
Lymphoma, Non-Hodgkin
Workup
Laboratory Studies
- A complete blood cell (CBC) count with differential demonstrates anemia and thrombocytopenia to varying degrees in individuals with acute lymphoblastic leukemia (ALL). Patients with ALL can have a high, normal, or low WBC count, but they usually exhibit neutropenia.
- Abnormalities in the prothrombin time (PT) / activated partial thromboplastin time (aPTT) / fibrinogen / fibrin degradation products may suggest concomitant DIC, which results in an elevated prothrombin time, decreased fibrinogen levels, and the presence of fibrin split products.
- A review of the peripheral blood smear confirms the findings of the CBC count.
- Circulating blasts are usually seen.
- Schistocytes are sometimes seen if DIC is present.
- A chemistry profile is recommended.
- Most patients with acute lymphoblastic leukemia (ALL) have an elevated lactic dehydrogenase level (LDH), and they frequently have an elevated uric acid level.
- Liver function tests and blood urea nitrogen (BUN)/creatinine determinations are necessary before the initiation of therapy.
- Appropriate cultures, in particular blood cultures, should be obtained in patients with fever or with other signs of infection without fever.
Imaging Studies
- Chest x-ray films may reveal signs of pneumonia and/or a prominent mediastinal mass in some cases of T-cell acute lymphoblastic leukemia (ALL).
- Computed tomography (CT) scans can further define the degree of lymphadenopathy in some patients, including those with mediastinal masses.
- Multiple-gated acquisition (MUGA) scans or electrocardiographs (ECGs) are needed when the diagnosis of acute lymphoblastic leukemia (ALL) is confirmed, because many chemotherapeutic agents used in the treatment of acute leukemia are cardiotoxic.
Other Tests
- An ECG is recommended before the initiation of treatment.
Procedures
- Bone marrow aspiration and biopsy are the definitive diagnostic tests to confirm the diagnosis of leukemia. Immunophenotyping helps elucidate the subtype.
- Aspiration slides should be stained for morphology with either Wright or Giemsa stain. The diagnosis of acute lymphoblastic leukemia (ALL) is made when at least 30% lymphoblasts (French-American-British [FAB] classification) or 20% lymphoblasts (World Health Organization [WHO] classification) are present in the bone marrow and/or peripheral blood.
- In addition, slides should be stained with myeloperoxidase (or Sudan black) and terminal deoxynucleotidyl transferase (TdT), unless another method is used, such as flow cytometry.
- Bone marrow samples should also be sent for cytogenetics and flow cytometry. Approximately 15% of patients with acute lymphoblastic leukemia (ALL) have a t(9;22) translocation (ie, Philadelphia [Ph] chromosome), but other chromosomal abnormalities may also occur, such as t(4;11), t(2;8), and t(8;14).
- A negative myeloperoxidase stain and a positive TdT is the hallmark of the diagnosis of most cases of acute lymphoblastic leukemia (ALL). However, positive confirmation of lymphoid (and not myeloid) lineage should be sought by flow cytometric demonstration of lymphoid antigens, such as CD3 (T-lineage ALL) or CD19 (B-lineage ALL), in order to avoid confusion with some types of myeloid leukemia (eg, M0), which also stain negative with myeloperoxidase.Although more than 95% of cases of the L1 or L2 subtype of acute lymphoblastic leukemia (ALL) are positive for TdT, TdT is not specific for ALL. TDT is absent in L3 (mature B-cell) ALL. TdT helps distinguish acute lymphoblastic leukemia (ALL) from malignancies of more mature lymphocytes (ie, non-Hodgkin lymphoma [NHL]).
- In cases of acute leukemia that are MPO negative, TdT positive, the distinction between AML and acute lymphoblastic leukemia (ALL) is made based on the analysis of flow cytometry results. Patients with AML demonstrate myeloid markers such as CD33, whereas patients with ALL demonstrate lymphoid markers. Further confusion arises because some patients with acute lymphoblastic leukemia (ALL) have aberrant expression of myeloid markers, such as CD13. However, if the cells are TdT positive, myeloperoxidase negative, CD33 negative and demonstrate lymphoid markers, the leukemia is considered ALL.
- Studies for bcr-abl analysis by polymerase chain reaction (PCR) or cytogenetics may help distinguish patients with Philadelphia chromosome–positive ALL from those with the lymphoid blastic phase of chronic myelogenous leukemia (CML). Most patients with Ph+ ALL have the p190 type of bcr-abl, whereas patients with lymphoid blastic CML have the p210 type of bcr-abl.
- Newer studies are analyzing acute lymphoblastic leukemia (ALL) subtypes by gene expression profiling. In children with ALL, Bogni et al distinguished 3 groups of patients.1 Interestingly, one of these groups had a significantly increased risk of developing treatment-related AML following chemotherapy for their acute lymphoblastic leukemia (ALL).
Histologic Findings
The older, traditional classification of acute lymphoblastic leukemia (ALL) is the FAB classification. This has now been replaced by the newer WHO classification but the FAB system is listed below for historical purposes:
- L1 – Small cells with homogeneous chromatin, regular nuclear shape, small or absent nucleolus, and scanty cytoplasm; subtype represents 25-30% of adult cases
- L2 – Large and heterogeneous cells, heterogeneous chromatin, irregular nuclear shape, and nucleolus often large; subtype represents 70% of cases (most common)
- L3 – Large and homogeneous cells with multiple nucleoli, moderate deep blue cytoplasm, and cytoplasmic vacuolization that often overlies the nucleus (most prominent feature); subtype represents 1-2% of adult cases
The WHO classifies the L1 and L2 subtypes of acute lymphoblastic leukemia (ALL) as either precursor B lymphoblastic leukemia/lymphoblastic lymphoma or precursor T lymphoblastic leukemia/lymphoblastic lymphoma depending on the cell of origin. The L3 subtype of ALL is included in the group of mature B-cell neoplasms, as the subtype Burkitt lymphoma/leukemia.
Cytogenetic abnormalities occur in approximately 70% of cases of acute lymphoblastic leukemia (ALL) in adults. These abnormalities include balanced translocations as occur in cases of AML. However, abnormalities of chromosome number (hypodiploidy, hyperdiploidy) are more common in ALL than in AML.
Table 1. Common Cytogenetic Abnormalities in ALL
Open table in new window
| Abnormality | Genes Involved | 3-Year Event-Free Survival |
t(10;14)(q24;q11) | HOX11/TCRA | 75% |
| 6q | Unknown | 47% |
| 14q11 | TCRA/TCRD | 42% |
| 11q23 | MLL | 18-26% |
| 9p | Unknown | 22% |
| 12 | TEL | 20% |
t(1;19)(q23;p13) | PBX1/E2A | 20% |
| t(8;14)(q24;q32) t(2;8)(p12;q24) t(8;22)(q24;q11) | c-myc/IGH IGK/c-myc c-myc/IGL | 17%* 80%† |
| t(9;22)(q34;q11) | bcr-abl | 5-10%* 66%‡ |
| t(4;11)(q21;q23) | AF4-MLL | 0-10% |
| * Traditional regimens. †Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin [Adriamycin], dexamethasone) with rituxan. ‡Hyper-CVAD with imatinib. | ||
Table 2. Effect of Chromosome Number on Prognosis
Open table in new window
| Chromosome Number | 3-Year Event-Free Survival |
| Near tetraploidy | 46-56% |
| Normal karyotype | 34-44% |
| Hyperdiploidy >50 | 32-59% |
| Hyperdiploidy 47-50 | 21-53% |
| Pseudodiploidy | 12-25% |
| Hypodiploidy | 11% |
Eighty-five percent of cases of ALL are derived from B cells. The primary distinction is between (1) early (pro-B) ALL, which is TDT positive, CD10 (CALLA) negative, surface Ig negative; (2) precursor B ALL, which is TDT positive, CD10 (CALLA) positive, surface Ig negative; and (3) mature B cell (Burkitt) ALL, which is TdT negative, surface Ig positive. Fifteen percent of cases are derived from T cells. These cases are subclassified into different stages corresponding to the phases of normal thymocyte development. The early subtype is surface CD3 negative, cytoplasmic CD3 positive, and either double negative (CD4-, CD8-) or double positive (CD4+, CD8+). The latter subtype is surface CD3 positive, CD1a negative, and positive for either CD4 or CD8, but not both.
Table 3. Immunophenotyping of ALL Cells – ALL of B-Cell Lineage (85% of cases of adult ALL)
Open table in new window
| ALL Cells | TdT | CD19 | CD10 | CyIg§ | SIg|| |
| Early B-precursor ALL | + | + | - | - | - |
| Pre–B-cell ALL¶ | + | + | + | + | - |
| B-cell ALL | - | + | +/- | +/- | + |
| § Cytoplasmic immunoglobulin. || Surface immunoglobulin. ¶ See image below. | |||||
Diagnostic workup of a patient with pre–B-cell acute lymphoblastic leukemia. Bone marrow aspiration revealed French-American-British L2 morphology.
Table 4. Immunophenotyping of ALL Cells – ALL of T-Cell Lineage (15% of cases of adult ALL)
Open table in new window
| ALL Cells | TdT | Surface CD3 | CD4/CD8 |
| Early T-precursor ALL | + | - | +/+ or -/- |
| T-cell ALL | + | + | +/- or -/+ |
Treatment & Medication
Treatment
Medical Care
Currently, only 20-30% of adults with acute lymphoblastic leukemia (ALL) are cured with standard chemotherapy regimens. Consequently, all patients must be evaluated for entry into well-designed clinical trials. If a clinical trial is not available, the patient can be treated with standard therapy. Traditionally, the 4 components of ALL treatment are induction, consolidation, maintenance, and central nervous system (CNS) prophylaxis. Other aspects of treatment are also discussed.
- Induction therapy
- Standard induction therapy typically involves either a 4-drug regimen of vincristine, prednisone, anthracycline, and cyclophosphamide or L -asparaginase or a 5-drug regimen of vincristine, prednisone, anthracycline, cyclophosphamide, and L -asparaginase given over the course of 4-6 weeks.
- Using this approach, complete remissions (CRs) are obtained in 65-85% of patients. The rapidity with which a patient's disease enters CR is correlated with treatment outcome.
- In a large French study, patients with greater than 5% blasts in their bone marrow on day 15 had a lower response rate (34% vs 91%), worse disease-free survival, and worse overall survival than patients with low blast counts on day 15.2
- Several other studies have shown that patients whose disease is in CR within 4 weeks of therapy have longer disease-free survival and overall survival than those whose disease enters remission after 4 weeks of treatment.
- Consolidation therapy
- The use of consolidation chemotherapy is supported by several studies. In 1987, Fiere et al compared consolidation therapy with daunorubicin and cytosine arabinoside (Ara-C) versus no consolidation therapy in adults with acute lymphoblastic leukemia (ALL).3 The 3-year, leukemia-free survival rate was 38% for subjects receiving consolidation and maintenance therapy compared with 0% for those receiving maintenance therapy without consolidation (P <0.05).
- In a 1984 study reported by Hoelzer et al, subjects whose disease was in remission after induction received consolidation therapy consisting of dexamethasone, vincristine, and doxorubicin, followed by cyclophosphamide, Ara-C, and 6-thioguanine beginning at week 20.4 Subjects also received maintenance therapy with 6-mercaptopurine and methotrexate during weeks 10-20 and 28-130. The median remission of 20 months was among the longest reported at the time.
- In the United Kingdom Acute Lymphoblastic Leukemia XA study, subjects were randomized to receive early intensification with Ara-C, etoposide, thioguanine, daunorubicin, vincristine, and prednisone at 5 weeks; late intensification with the same regimen at 20 weeks; both; or neither.5 The disease-free survival rates at 5 years were 34%, 25%, 37%, and 28%, respectively. These data suggest a benefit to early, rather than late, intensification.
- A study by the Cancer and Leukemia Group B (CALGB) did not show a benefit to consolidation therapy. Subjects whose disease was in CR were randomized to receive maintenance therapy or intensification with 2 courses of Ara-C and daunorubicin followed by maintenance. Remission duration and overall survival were not affected by the randomization.
- Because most studies have showed a benefit to consolidation therapy, regimens using a standard 4- to 5-drug induction usually include consolidation therapy with Ara-C in combination with an anthracycline or epipodophyllotoxin.
- Maintenance therapy
- The effectiveness of maintenance chemotherapy in adults with acute lymphoblastic leukemia (ALL) has not been studied in a controlled clinical trial. However, several phase 2 studies without maintenance therapy have shown inferior results compared with historical controls.
- A CALGB study of daunorubicin or mitoxantrone, vincristine, prednisone, and methotrexate induction followed by 4 intensifications and no maintenance was closed early because the median remission duration was shorter than in previous studies.6 A Dutch study using intensive postremission chemotherapy, 3 courses of high-dose Ara-C in combination with amsacrine (course 1), mitoxantrone (course 2), and etoposide (course 3), without maintenance, also yielded inferior results.7
- Although maintenance appears necessary, using a more intensive versus less intensive regimen does not appear to be beneficial. Intensification of maintenance therapy from a 12-month course of a 4-drug regimen compared with a 14-month course of a 7-drug regimen did not show a difference in disease-free survival between the 2 groups.8
- CNS prophylaxis
- In contrast to patients with AML, patients with acute lymphoblastic leukemia (ALL) frequently have meningeal leukemia at the time of relapse. A minority of patients have meningeal disease at the time of initial diagnosis. As a result, CNS prophylaxis with intrathecal chemotherapy is essential.
- Cortes et al analyzed the prevalence of CNS leukemia in 4 consecutive clinical trials at the M.D. Anderson Cancer Center.9
- In the first group, subjects received standard systemic chemotherapy without CNS prophylaxis. In the second, subjects received high-dose systemic chemotherapy and no CNS prophylaxis. The third group received high-dose systemic chemotherapy and intrathecal chemotherapy for high-risk subjects after achieving remission. The fourth group received hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (ie, hyper-CVAD protocol).
- All subjects received intrathecal chemotherapy starting in induction. High-risk subjects received 16 intrathecal treatments, and low-risk subjects received 4 intrathecal treatments.
- Overall, CNS relapse rates were 31% for group 1 (standard chemotherapy, no CNS prophylaxis), 18% for group 2 (high-dose systemic chemotherapy, no CNS prophylaxis), 17% for group 3 (high-dose systemic chemotherapy, intrathecal chemotherapy), and 3% for group 4 (hyper-CVAD).
- This study demonstrated that high-dose systemic chemotherapy reduces CNS relapse; however, early intrathecal chemotherapy is necessary to achieve the lowest risk of CNS relapse.
- Newer approaches
- Standard induction regimens are modeled after pediatric programs and were originally developed when supportive care was significantly inferior to what is available today. Few antibiotics were available, and transfusion capabilities were minimal. Consequently, milder regimens were designed in an attempt to minimize early deaths during induction.
- With the addition of third-generation cephalosporins and sophisticated blood-banking techniques, the ability to support patients through a pancytopenic phase has increased dramatically. As a result, more intensive induction approaches are used by many physicians. Two notable examples are the Memorial ALL-2 protocol and the hyper-CVAD protocol.
- The ALL-2 protocol uses an intensive, high-dose, mitoxantrone-based, AML-style induction regimen. In a phase 1 study of high-dose mitoxantrone combined with high-dose Ara-C, Arlin et al reported that 8 of 8 patients newly diagnosed with acute lymphoblastic leukemia (ALL) and 8 of 10 patients with ALL who relapsed achieved CR.10
- In 1996, Weiss et al reported treatment of 37 subjects with newly diagnosed acute lymphoblastic leukemia (ALL) with this induction regimen followed by a first consolidation with vincristine, prednisone, L -asparaginase, and methotrexate; a second consolidation with Ara-C and etoposide; and then 2 years of maintenance therapy.11 Of these subjects, 84% achieved CR. The median remission duration was 17 months, and median survival was 20 months.
- In a randomized phase III trial comparing the ALL-2 regimen with the L-20 regimen, the CR rate was 83% for patients receiving ALL-2 compared with 70% for patients receiving L-20 (P = 0.05). Overall survival at 4 years was superior for patients receiving ALL-2 (40%) versus those receiving L-20 (22%).
- The hyper-CVAD regimen is based on the success achieved with short-term, dose-intensive chemotherapy regimens in children. It incorporates hyperfractionated cyclophosphamide and intensive doses of Ara-C and methotrexate in combination with dexamethasone and vincristine. Maintenance therapy with prednisone, vincristine (Oncovin), methotrexate, and mercaptopurine (Purinethol) (ie, POMP protocol) is given to patients with nonmature B-cell ALL.
- From 1992-2000, 288 patients received hyper-CVAD at M.D. Anderson Cancer Center. The Philadelphia chromosome was present in 17% of patients, and 13% had T-cell ALL. Overall, 92% of patients obtained a CR. The 5-year survival and percentage of patients in CR at 5 years were both 38%. Patients with Ph+ ALL had a 92% CR rate but only a 12% 5-year survival. Patients with T-cell ALL had a 75% CR rate and a 48% 5-year survival. Patients with Burkitt ALL had a 93% CR rate and a 67% 5-year survival.
- Newer modifications of the hyper-CVAD regimen include the addition of imatinib to patients whose leukemia is Philadelphia chromosome positive, and rituxan to patients whose leukemia is CD20 positive. Both of these approaches have resulted in improvements in disease-free survival (see below).
- Treatment of mature B-cell ALL
- Mature B-cell acute lymphoblastic leukemia (ALL) is a special type, representing only 5% of adult patients with ALL. The hallmark of mature B-cell ALL is the presence of surface immunoglobulin on the lymphoblasts. Using conventional regimens, only 30-40% of patients enter CR and few patients survive long term.
- Newer short-term intensive therapies are showing improved results. A report of the hyper-CVAD regimen showed that disease in 93% of subjects entered CR, median survival was 16 months, and disease in 67% of subjects alive at 5 years.
- In a report by Hoelzer et al, with the use of regimens containing intensive cyclophosphamide and intermediate methotrexate or ifosfamide and high-dose methotrexate, CR rates were 63% (cyclophosphamide + intermediate methotrexate) and 74% (ifosfamide + high-dose methotrexate).12
- Disease-free survival rates increased to 50% in the first group and 71% in the second group, and overall survival increased to 50% compared with 0% for historical controls. Although previously these patients were referred for transplantation in first remission, many physicians now defer transplantation for the time of relapse because of these improved results.
- Burkitt ALL cells are CD20 positive. This allows for the addition of targeted therapy with rituximab. Many studies are have demonstrated improved efficacy, including prolonged survival, when rituximab is added to chemotherapy in these patients. The combination of hyper-CVAD plus rituxan resulted in an overall 3 year survival of 80% compared with 50% for historical controls treated without rituxan.13
- Treatment of Philadelphia chromosome–positive ALL
- In the past, Ph+ ALL was treated with the same regimens as other types of acute lymphoblastic leukemia (ALL), with poor results. However, imatinib inhibits the bcr-abl fusion protein of Ph+ ALL and thus allows targeted therapy of this disease. As a single agent, imatinib has limited activity.
- In an early study of patients with Ph+ ALL or CML in lymphoid blast crisis, only 4 of 20 patients had a complete response, and all patients progressed in less than 6 months.
- The German Multicenter ALL (GMALL) trial conducted a randomized study of imatinib versus standard induction therapy for patients with Philadelphia chromosome–positive ALL older than age 55 years.14 The overall CR rate was 96.3% in patients randomly assigned to imatinib and 50% in patients allocated to standard chemotherapy (P = 0.0001). Severe adverse events were significantly more frequent during standard induction chemotherapy (90% vs 39%; P = .005). The estimated overall survival of all patients was 42% at 24 months, with no significant difference between the 2 cohorts.
- The addition of imatinib to chemotherapy has resulted in significantly improved outcomes. The addition of imatinib to hyper-CVAD resulted in a 3-year disease-free survival rate of 66% and overall survival of 55% compared with a 14% 3-year disease-free survival rate and 15% overall survival for patients treated with hyper-CVAD without imatinib.15 Similar results have been reported when imatinib is added to other chemotherapy regimens.
- Newer tyrosine kinase inhibitors have been developed for patients with CML that has become resistant to imatinib. These agents are also being studied in Philadelphia chromosome–positive ALL.
- Nilotinib is a new tyrosine kinase inhibitor that has a higher binding affinity and selectivity for the ABL kinase than imatinib.16 Nilotinib has 20 to 50 times the inhibitory activity against imatinib-sensitive CML cell lines relative to imatinib. In a phase II study in patients with relapsed/refractory Philadelphia chromosome positive ALL, complete responses were reported in 10 (24%) patients treated with nilotinib.16
- Dasatinib is a potent, orally active inhibitor of the BCR-ABL, c-KIT and the SRC family of kinases.17Dasatinib is a more potent inhibitor of BCR-ABL and c-KIT than imatinib mesylate, and it is effective in patients with CML that is resistant to or intolerant of imatinib.
- The Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) presented the interim results of a prospective study of dasatinib in patients with newly diagnosed Philadelphia chromosome–positive ALL. Prednisone was started 7 days before the first dasatinib administration and continued until day 31. Dasatinib was administered for a total of 84 days. At the time of the report, all 23 patients treated showed a complete hematologic response by day +22.
- Although nilotinib and dasatinib are clearly active in Philadelphia chromosome–positive ALL, it is likely that, similar to the results seen with imatinib, these responses will likely not be durable. Therefore each of these agents is currently being studied in combination with standard chemotherapy regimens.
- Treatment of the younger adult
- Older children and younger adults with acute lymphoblastic leukemia (ALL) can be referred to either adult or pediatric hematologists. Usually, the patient will receive either an adult or pediatric regimen based on this referral pattern. However, several studies have suggested that younger patients are best treated on pediatric protocols.
- For example, in a retrospective analysis of patients aged 15-20 years treated on either the FRALLE 93 or LALA 94 trials, the CR rate was 94% for patients receiving the pediatric regimen compared with 83% for those receiving the adult regimen (p = 0.04).18 The 5-year survival was 67% in the pediatric-regimen group and 41% in the adult-regimen group (P <0.0001). Patients treated on the pediatric regimen were younger (15.9 y) than those treated on the adult regimen (17.9 y); however, prognostic factors were otherwise matched.
- Similarly, the Children’s Cancer Group (CCG) and CALGB performed an analysis on patients aged 16-21 years treated on their studies.19 Again, event-free and overall survival were improved for patients treated on the CCG protocols.
- In a study by the Programme for the Study of Therapeutics for Haematological Malignancies (PETHEMA), adolescents and young adults were treated with a pediatric regimen (ALL-96).20 The response to therapy was the similar to previously reported, although a slight increase in hematologic toxicity was observed in the adult patients.
- Other lessons learned from pediatric protocols
- The majority of children with acute lymphoblastic leukemia (ALL) are cured with frontline chemotherapy regimens. Many investigators are trying to translate these results into the adult population. Areas being studied include increased intensity of standard agents including asparaginase, risk-adapted chemotherapy, and evaluation of minimal disease.
- Transplantation
- Relatively few studies have compared transplantation with chemotherapy in adults with acute lymphoblastic leukemia (ALL). In a study by the Groupe Ouest-Est des Leucemies Airgues et Maladies du Sang (GOELAMS), subjects younger than 45 years who had a sibling donor and whose disease was in remission were assigned to allogeneic transplantation.21 The remaining subjects received methylprednisolone, Ara-C, mitoxantrone, and etoposide chemotherapy followed by autologous bone marrow transplantation (BMT).
- For subjects undergoing allogeneic BMT, the rate of freedom from relapse was 70% at 4 years. However, because of transplant-related complications, the event-free survival rate was only 33%. No toxic deaths occurred in the subjects who underwent autologous BMT. However, the event-free survival rate was only 17% at 4 years because of a high rate of relapse.
- The Bordeaux, Grenoble, Marseille, Toulouse group conducted a prospective nonrandomized trial comparing allogeneic with autologous BMT and also tested the impact of recombinant interleukin 2 (IL-2) after autologous BMT. The treatment arm was selected based on the availability of an human leukocyte antigen (HLA)-matched sibling donor.
- The 3-year probability of disease-free survival was significantly higher in the group assigned to allogeneic BMT (68%) compared with subjects assigned to autologous BMT (26%, P <0.001). No benefit was observed with the addition of IL-2 after autologous BMT.
- In the French Group on Therapy for Adult Acute Lymphoblastic Leukemia 1987 study, subjects aged 15-40 years whose disease was in CR and who had an HLA-compatible sibling donor underwent allogeneic BMT.2 The other subjects were randomized to receive autologous BMT or chemotherapy. Overall, no difference in was observed in 5-year survival between the groups.
- When only high-risk patients were considered (ie, Ph+, null ALL, >35 y, WBC count >30,000/µL, or time to CR > 4 wk), overall survival rates (44% vs 20%) and disease-free survival rates (39% vs 14%) were superior for those undergoing allogeneic BMT versus those undergoing either autologous BMT or chemotherapy. Other phase 2 studies have confirmed a benefit for high-risk patients who undergo allogeneic BMT, with as many as 50% achieving long-term remissions.
- In the GOELAL02 study, patients with any high-risk feature (age >35 y, non–T-ALL, WBC >30,000, adverse cytogenetics: t[9;22], t[4;11], or t[1;19], or no CR after induction) received either allogeneic or autologous stem cell transplantation. For patients younger than 50 years, the 6-year overall survival rate was improved for patients receiving allogeneic transplantation (75%) compared with those receiving autologous transplantation (40%) (P = 0.0027).21
- The United Kingdom Medical Research Council Acute Lymphoblastic Leukemia joint trial with the Eastern Cooperative Oncology Group (MRC UKALL XII/ECOG E2993) evaluated the role of allogeneic transplantation for adults with ALL and compared autologous transplantation with standard chemotherapy.22 Patients received 2 phases of induction and, if in remission, were assigned to allogeneic transplantation if they had a compatible sibling donor. Other patients were randomized to chemotherapy for 2.5 years versus an autologous transplantation.
- A donor versus no-donor analysis showed that Philadelphia chromosome–negative patients with a donor had a 5-year improved overall survival, 53% versus 45% (P = 0.01), and that the relapse rate was significantly lower. The survival difference was significant in standard-risk patients but not in high-risk patients with a high nonrelapse mortality rate in the high-risk donor group. Patients randomized to chemotherapy had a higher 5-year overall survival (46%) than those randomized to autologous transplantation (37%; P = 0.03).
- This study demonstrated that matched related allogeneic transplantations for acute lymphoblastic leukemia (ALL) in first complete CR provide the most potent antileukemic therapy and considerable survival benefit for standard-risk patients. However, the transplantation-related mortality for high-risk older patients was unacceptably high and abrogated the reduction in relapse risk.
- Allogeneic transplantation can also be effective therapy for patients who have experienced relapse after chemotherapy. Martino et al treated 37 consecutive patients with primary refractory or relapsed acute lymphoblastic leukemia (ALL) with intensive salvage chemotherapy.23
- Of the 29 patients whose disease went into CR, 10 were assigned to allogeneic BMT based on the availability of a sibling donor. The remaining 19 patients were assigned to autologous BMT.
- Of the 19 patients assigned to autologous BMT, 10 did not reach transplantation, mostly because of early relapse; 9 received transplants. Of these, 1 died early and 8 experienced relapse 2-30 months after transplantation.
- Of the 10 patients who received allogeneic BMT, 4 died early and 6 were alive and free from disease 9.7-92.6 months after the transplantation.
- These results are similar to those in patients in earlier stages, indicating that transplant-related complications are increased in the allogeneic setting. However, a significant number of patients can be cured. Yet, although autologous transplantation is relatively safe, it is associated with a high relapse rate, making this modality of little use in patients with acute lymphoblastic leukemia (ALL).
- For patients without a sibling donor, an alternative is an unrelated donor (URD) transplant. Weisdorf et al compared the results of 6 years of consecutive autologous transplantations (n = 214) with URD transplantations from the National Marrow Donor Program (n = 337).24 Autologous BMT was associated with a lower transplant-related mortality rate. However, URD transplantations had a lower risk of relapse. In patients whose disease was in second CR, URD transplantations resulted in a superior rate of disease-free survival.
- In summary, most authorities agree that allogeneic transplantation should be offered to young patients with high-risk features whose disease is in first remission. Young patients without adverse features should receive induction, consolidation, and maintenance therapy. In these patients, transplantation is reserved for relapse.
- Older patients whose disease is in CR may be considered for such investigational approaches as allogeneic transplantation with nonmyeloablative chemotherapy (ie, minitransplants). Although previously patients with mature B-cell ALL would have been referred for transplantation when their disease was in first CR with improving results from more intensive chemotherapy regimens, many clinicians are reserving transplantation for patients who have experienced relapse.
- Hematopoietic stem cell transplantation (HSCT) seems to be a valuable option for a subgroup of infants with mixed-lineage (MLL +) acute lymphoblastic leukemia carrying poor prognostic factors that include age younger than 6 months and either poor response to steroids at day 8 or leukocytes more than or equal to 300 g/L.25
- Treatment of relapsed ALL
- Patients with relapsed acute lymphoblastic leukemia (ALL) have an extremely poor prognosis. Most patients are referred for investigational therapies. Young patients who have not previously undergone transplantation are referred for such therapy. Reinduction regimens include the hyper-CVAD protocol and high-dose Ara-C–based regimens.
- As noted above, the hyper-CVAD regimen is based on hyperfractionated cyclophosphamide and intermediate doses of Ara-C and methotrexate. In a study at the M.D. Anderson Cancer Center of 66 patients with relapsed ALL, the CR rate was 44% and median survival was 42 weeks.
- Arlin et al reported that 8 of 10 patients with relapsed acute lymphoblastic leukemia (ALL) achieved CR with high-dose Ara-C and high-dose mitoxantrone.10 A similar regimen using a single high dose of idarubicin in combination with Ara-C (the Memorial ALL-3 protocol) resulted in CR rates of 58-78% in patients who experienced relapse.
- In the Italian ALL R-87 study, 61 subjects with acute lymphoblastic leukemia (ALL) in first relapse received induction chemotherapy with intermediate-dose Ara-C, idarubicin, and prednisone.
- Subjects whose disease was in remission were to receive consolidation chemotherapy and then BMT. Of these subjects, 56% achieved CR; however, only 9 of the responders underwent BMT.
- The remaining subjects did not undergo transplantations because of either early relapse or excessive toxicity.
- Of the 4 subjects who underwent allogeneic BMT, 3 were alive and achieved remission at 22, 43, and 63 months, whereas only 1 of the 5 subjects who underwent autologous BMT was alive.
- This study suggests that a small number of patients who experience relapse will survive long-term after allogeneic BMT. However, autologous BMT is less useful because it is associated with a high rate of relapse.
- Newer drugs
- A number of new drugs are currently in development for the treatment of acute lymphoblastic leukemia (ALL). A few examples are as follows.
- Clofarabine is a novel nucleoside analogue that is approved for the treatment of pediatric patients with refractory or relapsed acute lymphoblastic leukemia (ALL).26 Clofarabine inhibits DNA synthesis at both DNA polymerase I and at RNA reductase. Overall response rates average 25%.
- 506U78 (nelarabine [Arranon]) is a novel purine nucleoside that is a prodrug of ara-G.27 It was approved as an orphan drug by the US Federal Drug Administration (FDA) in October 2005. Complete responses are reported in 31% of patients and in 54% of patients with T-cell ALL. The dose-limiting toxicity of this drug is neurotoxicity.
- Supportive care with replacement of blood products
- Patients with acute lymphoblastic leukemia (ALL) have a deficiency in the ability to produce normal blood cells, and they need replacement therapy. This deficiency is temporarily worsened by the addition of chemotherapy. All blood products must be irradiated to prevent transfusion-related graft versus host disease, which is almost invariably fatal.
- Packed red blood cells are given to patients with a hemoglobin level of less than 7-8 g/dL or at a higher level if the patient has significant cardiovascular or respiratory compromise.
- Platelets are transfused if the count is less than 10,000-20,000/µL. Patients with pulmonary or gastrointestinal hemorrhage receive platelet transfusions to maintain a value greater than 50,000/µL. Patients with CNS hemorrhage are transfused to achieve a platelet count of 100,000/µL.
- Fresh frozen plasma is given to patients with a significantly prolonged PT, and cryoprecipitate is given if the fibrinogen level is less than 100 g/dL.
- Supportive care with antibiotics
- These are given to all febrile patients. At a minimum, include a third-generation cephalosporin (or equivalent), usually with an aminoglycoside. In addition to this minimum, other antibiotics are added to treat specific documented or possible infections.
- Patients with persistent fever after 3-5 days of antibacterial antibiotics should have an antifungal antibiotic (liposomal or lipid complex amphotericin, new generation azole or echinocandin) added to their regimen. Patients with sinopulmonary complaints would receive anti-Aspergillus treatment. Particular care is warranted for patients receiving steroids as part of their treatment, because the signs and symptoms of infection may be subtle or even absent.
- The use of prophylactic antibiotics in neutropenic patients who are not febrile is controversial. However, most clinicians prescribe them for patients undergoing induction therapy. A commonly used regimen includes the following:
- Ciprofloxacin (500 mg PO bid)
- Fluconazole (200 mg PO daily), itraconazole (200 mg PO bid), or posaconazole (200 mg PO tid)
- Acyclovir (200 mg PO 5 times/d) or valacyclovir (500 mg PO daily)
- Once patients taking these antibiotics become febrile, they are switched to intravenous antibiotics as described above.
- Supportive care with growth factors
- The use of granulocyte colony-stimulating factor (G-CSF) during induction chemotherapy is supported by several studies. In a randomized phase 3 trial conducted by Ottoman, 76 subjects received either G-CSF or no growth factor with the induction chemotherapy (ie, cyclophosphamide, Ara-C, 6-mercaptopurine, intrathecal methotrexate, and cranial irradiation). The median duration of neutropenia was 8 days in subjects receiving G-CSF versus 12 days in subjects receiving no growth factor (P <0.002), and the prevalence of nonviral infections was decreased by 50% in subjects receiving G-CSF. No difference in disease-free survival was observed between the 2 group.
- In a randomized phase 3 study reported by Geissler et al, 53 subjects received either G-CSF beginning on day 2 of induction chemotherapy (ie, with daunorubicin, vincristine, L -asparaginase, and prednisone) or chemotherapy without G-CSF.28 G-CSF markedly decreased the proportion of days with neutropenia of less than 1000/µL (29% for G-CSF vs 84% for controls, P <0.00005), reduced the prevalence of febrile neutropenia (12% vs 42% in controls, P <0.05), and decreased the prevalence of documented infections (40% vs 77%, P <0.05). No difference was observed in response, remission duration, or survival between the 2 groups.
- In the CALGB 9111 study, 198 subjects were randomized to receive either placebo or G-CSF beginning on day 4 of induction chemotherapy.29 Again, subjects in the G-CSF group had significantly shorter durations of neutropenia and significantly fewer days of hospitalization. In this study, subjects receiving G-CSF also had higher CR rates because fewer deaths occurred during remission induction. Again, no significant effect on disease-free survival or overall survival was observed.
- The importance of the early use of G-CSF is demonstrated by the study of Bassan et al, in which subjects received induction chemotherapy with idarubicin, vincristine, L -asparaginase, and prednisone.30 Twenty-eight subjects received G-CSF beginning on day 15, and 37 subjects received G-CSF beginning on day 4. Subjects receiving G-CSF on day 4 recovered significantly faster from neutropenia, had fewer infectious complications, and required less antibiotic than subjects beginning G-CSF on day 15.
- Currently, outside of the setting of a clinical trial, few data support the use of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with acute lymphoblastic leukemia (ALL). The GOELAMS investigators randomly assigned 67 subjects to receive GM-CSF or placebo during induction chemotherapy with idarubicin, methylprednisolone, and high-dose Ara-C.31 No difference was observed in the CR rate, the duration of neutropenia, or days with fever for the 2 groups.
- Mucositis of higher than grade 3 was reduced in subjects receiving GM-CSF (2 of 35 patients vs 6 of 29 patients, respectively, P = 0.03).31 In a Groupe d'Etude et de Traitement de la Leucemie Aigue Lymphoblastique de l'Adulte (GET-LALA) study, patients received G-CSF, GM-CSF, or no growth factor during induction therapy.32 The median time for neutrophil recovery was 17 days for G-CSF, 18 days for GM-CSF, and 21 days for no growth factors.
- Allopurinol at 300 mg 1-3 times per day is recommended during induction therapy until blasts are cleared and hyperuricemia resolves. High-risk patients (those with very high LDH or leukemic infiltration of the kidneys) can benefit from rasburicase.
Surgical Care
Placement of a central venous catheter, such as a triple lumen, Broviac, or Hickman catheter, may be necessary.
Diet
A neutropenic diet is recommended in those with acute lymphoblastic leukemia (ALL).
- No fresh fruits or vegetables may be eaten.
- All foods must be cooked.
- Meats are to be cooked until well done.
Activity
Activity may occur as tolerated by the patient, but the patient may not participate in strenuous activities such as lifting or exercise.
Medication
The medications used to treat acute leukemia cause severe bone marrow depression. Only physicians specifically trained in their use should administer these medications. In addition, access to appropriate supportive care is required.
A regimen of fludarabine and cyclophosphamide and rituximab (FCR) is commonly used. This combination of 3 of the most active drugs is a good example of a synergistic combination of drugs with different mechanisms. Different forms of the FCR combination are used; therefore, the dosing plays a big role in results. FCR provides a cyclical form of treatment, rather than a one-time, transplant-conditioning regimen followed by stem cells. In a study by Matutes et al, the FCR regimen did not quite overcome fludarabine resistance, which remains probably the single most important hurdle in long-term term outcomes for patients with acute lymphoblastic leukemia (ALL).33
Corticosteroids
Corticosteroids may be used during induction, consolidation, and/or maintenance therapy.
Prednisone (Deltasone, Orasone, Sterapred)
Has a wide range of activities. In ALL, used because of direct antileukemic effects.
Adult
60 mg/m2 PO qd for 28 d during induction; followed by 10-d taper
Pediatric
Not established
Antineoplastics
Antineoplastics are used for induction, consolidation, maintenance, and CNS prophylaxis.
Vincristine (Oncovin, Vincasar)
Vinca alkaloid that acts by arresting cells in metaphase.
Adult
2 mg/m2 IV push qwk for 5 wk during induction
Most cap the vincristine dose at 4 mg for young patients and 2.5 mg for older patients
Most cap the vincristine dose at 4 mg for young patients and 2.5 mg for older patients
Pediatric
Not established
Asparaginase (Elspar)
Breaks down extracellular asparagine into aspartic acid and ammonia. Normal cells are capable of synthesizing their own asparagine but many malignant cells are not.
Adult
6,000-12,000 U/m2 IM
Pediatric
Not established
Methotrexate (Folex, Rheumatrex)
Antimetabolite of folic acid analogue type. Inhibits dihydrofolate reductase, resulting in inhibition of DNA synthesis, repair, and cellular replication.
Adult
15 mg/m2 PO qwk during maintenance therapy
Pediatric
Not established
Mercaptopurine (Purinethol)
Antimetabolite of purine analogue type. Primary effect is inhibition of DNA synthesis.
Adult
100 mg/m2 PO qd during maintenance
Pediatric
Not established
Cyclophosphamide (Cytoxan)
Alkylating agent of nitrogen mustard type. Inhibits cell growth and proliferation.
Adult
1 g/m2 IV during induction
Pediatric
Not established
Cytosine arabinoside (Cytosar-U)
Antimetabolite that induces activity as a result of activation to cytarabine triphosphate and includes inhibition of DNA polymerase and incorporation into DNA and RNA.
Adult
100 mg/m2 IV as 24-h infusion qd for 7 d
3 g/m2 IV as 3-h infusion qd for 5 d
3 g/m2 IV as 3-h infusion qd for 5 d
Pediatric
Not established
Daunorubicin (Cerubidine)
Anthracycline that inhibits topoisomerase II. Also inhibits DNA and RNA synthesis by intercalating between DNA base pairs.
Adult
45-60 mg/m2 IV as a 30-min infusion qd for 3 d
Pediatric
Not established
Idarubicin (Idamycin)
Topoisomerase II inhibitor. Inhibits cell proliferation by inhibiting DNA and RNA polymerase.
Adult
12 mg/m2 IV as 30-min infusion qd for 3 d
Pediatric
Not established
Mitoxantrone (Novantrone)
Topoisomerase II inhibitor. Inhibits cell proliferation by intercalating DNA and inhibiting topoisomerase II.
Adult
12 mg/m2 IV as 30-min infusion qd for 3 d
Pediatric
Not established
Dasatinib (Sprycel)
Multiple tyrosine kinase inhibitor. Inhibits growth of cell lines overexpressing BCR -ABL.
Indicated for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in individuals resistant to or intolerant of previous therapy.
Indicated for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in individuals resistant to or intolerant of previous therapy.
Adult
140 mg PO qd; continue until disease progression or no longer tolerated
May increase to 180 mg PO qd if inadequate response
Coadministration with strong CYP3A4 inhibitors: decrease to 40 mg/d if taking 140 mg/d
Coadministration with CYP3A4 inducers: May need to increase dose (monitor for toxicity)
If clinically viable, an alternate medication with no or minimal enzyme inhibition or induction is recommended
May increase to 180 mg PO qd if inadequate response
Coadministration with strong CYP3A4 inhibitors: decrease to 40 mg/d if taking 140 mg/d
Coadministration with CYP3A4 inducers: May need to increase dose (monitor for toxicity)
If clinically viable, an alternate medication with no or minimal enzyme inhibition or induction is recommended
Pediatric
Not established
Nelarabine (Arranon)
Prodrug of the deoxyguanosine analogue 9-beta-D-arabinofuranosylguanine (ara-G). Converted to the active 5'-triphosphate, ara-GTP, a T-cell–selective nucleoside analogue. Leukemic blast cells accumulate ara-GTP. This allows for incorporation into DNA, leading to inhibition of DNA synthesis and cell death.
Approved by FDA as orphan drug to treat persons with T-cell acute lymphoblastic leukemia whose disease has not responded to or which has relapsed with at least 2 chemotherapy regimens.
Approved by FDA as orphan drug to treat persons with T-cell acute lymphoblastic leukemia whose disease has not responded to or which has relapsed with at least 2 chemotherapy regimens.
Adult
1500 mg/m2 IV (infuse over 2 h) on days 1, 3, and 5; repeat q21d
Pediatric
650 mg/m2 IV (infuse over 1 h) qd for 5 consecutive days; repeat q21d
Colony-Stimulating Factors
Colony-stimulating factors act as hematopoietic growth factors that stimulate development of granulocytes. These agents are used to treat or prevent neutropenia when patients receive myelosuppressive cancer chemotherapy and to reduce the period of neutropenia that is associated with BMT. Colony-stimulating factors are also used to mobilize autologous peripheral blood progenitor cells for BMT and in management of chronic neutropenia.
Filgrastim (Neupogen)
G-CSF that activates and stimulates production, maturation, migration, and cytotoxicity of neutrophils.
Adult
5 mcg/kg/d SC
Pediatric
Not established
Pegfilgrastim (Neulasta)
Long-acting filgrastim created by covalent conjugate of recombinant G-CSF (ie, filgrastim) and monomethoxypolyethylene glycol. As with filgrastim, acts on hematopoietic cells by binding to specific cell surface receptors, thereby activating and stimulating production, maturation, migration, and cytotoxicity of neutrophils.
Adult
6 mg SC once per chemotherapy cycle
Pediatric
<45 kg: Not established
>45 kg: Administer as in adults.
>45 kg: Administer as in adults.
Follow-up
Further Inpatient Care
- Patients with acute lymphoblastic leukemia (ALL) require admission for induction chemotherapy, and they require readmission for consolidation chemotherapy or for the treatment of toxic effects of chemotherapy.
Further Outpatient Care
- Maintenance therapy is administered in an outpatient setting.
- Patients come to the office to be monitored for disease status and the effects of chemotherapy.
Transfer
- Patients with acute lymphoblastic leukemia (ALL) are best treated at a center with personnel who have significant experience in the treatment of leukemia.
- Patients admitted to hospitals that lack appropriate blood product support facilities, leukapheresis capabilities, or physicians and nurses familiar with the treatment of patients with leukemia should be transferred to an appropriate (generally tertiary care) hospital.
Deterrence/Prevention
- While taking chemotherapy, patients with leukemia should avoid exposure to crowds and people with contagious illnesses, especially children with viral infections.
Complications
- Death in those with acute lymphoblastic leukemia (ALL) may occur as a result of uncontrolled infection or hemorrhage. This may occur even after the use of appropriate blood product and antibiotic support.
- The most common complication is failure of the leukemia to respond to chemotherapy. These patients do poorly because they usually do not respond to other chemotherapy regimens.
Prognosis
- Patients with acute lymphoblastic leukemia (ALL) are divided into the following 3 prognostic groups:
- Good risk includes (1) no adverse cytogenetics, (2) age younger than 30 years, (3) WBC count of less than 30,000/μL, and (4) complete remission within 4 weeks.
- Intermediate risk includes those whose condition does not meet the criteria for either good risk or poor risk.
- Poor risk includes (1) adverse cytogenetics [(t9;22), (4;11)], (2) age older than 60 years, (3) precursor B-cell WBCs with WBC count greater than 100,000/μL, or (4) failure to achieve complete remission within 4 weeks.
- Patients with precursor B-cell ALL have an extremely poor prognosis. Essentially, following standard chemotherapy or autologous transplantation, long-term survival is not achieved. Several reports have indicated that some patients with precursor B-cell ALL and t(4;11) may have prolonged survival following allogeneic transplantation; therefore, this is the treatment of choice.
- The effect of immunophenotype on prognosis are as follows:
- Czuczman et al studied 259 patients treated with several CALGB protocols for newly diagnosed acute lymphoblastic leukemia (ALL).34 B-lineage phenotype was expressed in 79% of patients; one third of these coexpressed myeloid antigens. Seventeen percent of patients demonstrated T-lineage ALL; one quarter of these coexpressed myeloid antigens. No significant difference in response rates, remission duration, or survival was observed for patients expressing myeloid antigens versus those not expressing myeloid antigens. T-lineage acute lymphoblastic leukemia (ALL) was associated with younger age, male sex, presence of a mediastinal mass, higher WBC count and hemoglobin level, longer survival, and longer disease-free survival. The number of T markers expressed also had prognostic significance. Patients expressing 6 or more markers had longer disease-free and overall survival compared with patients expressing 3 or fewer markers.
- In a report by Preti et al, 64 of 162 patients with newly diagnosed acute lymphoblastic leukemia (ALL) coexpressed myeloid markers.35 Patients coexpressing myeloid markers were significantly older, had a higher prevalence of CD34 expression, and had a lower prevalence of common ALL antigen expression than patients without myeloid expression. A trend toward a decreased remission rate was observed for patients coexpressing myeloid markers (64%) as compared with those who did not coexpress such markers (78%) (P = 0.06). However, no significant effect on remission duration or overall survival was observed.
- The effect of chromosome number on prognosis is displayed in Table 2.
Patient Education
- Patients with acute lymphoblastic leukemia (ALL) should be instructed to immediately seek medical attention if they are febrile or have signs of bleeding.
Miscellaneous
Medicolegal Pitfalls
- Patients with acute lymphoblastic leukemia (ALL) are best treated by physicians who have significant experience in the treatment of patients with acute leukemia. In addition, these patients should be treated in a setting where appropriate supportive care measures (high-level blood banking and leukapheresis) are available.